- KEY POINTS:
- Clinical update of newly-diagnosed unmethylated MGMT GBM
- Virtual recorded oral presentation (10 minutes)
- Highlights include PFS and OS benefits, with a significant number of patients treated with 10 or greater cycles
- PK summary of CSF concentration
- Case study of patient with >39 months OS
Hello, I am Dr. Chen. On behalf of our study team, I'm pleased to present our work, Phase 2 Study of VAL-083 and radiotherapy in newly-diagnosed MGMT-unmethylated glioblastoma. Thanks to the organizer for giving us this opportunity to present our work.
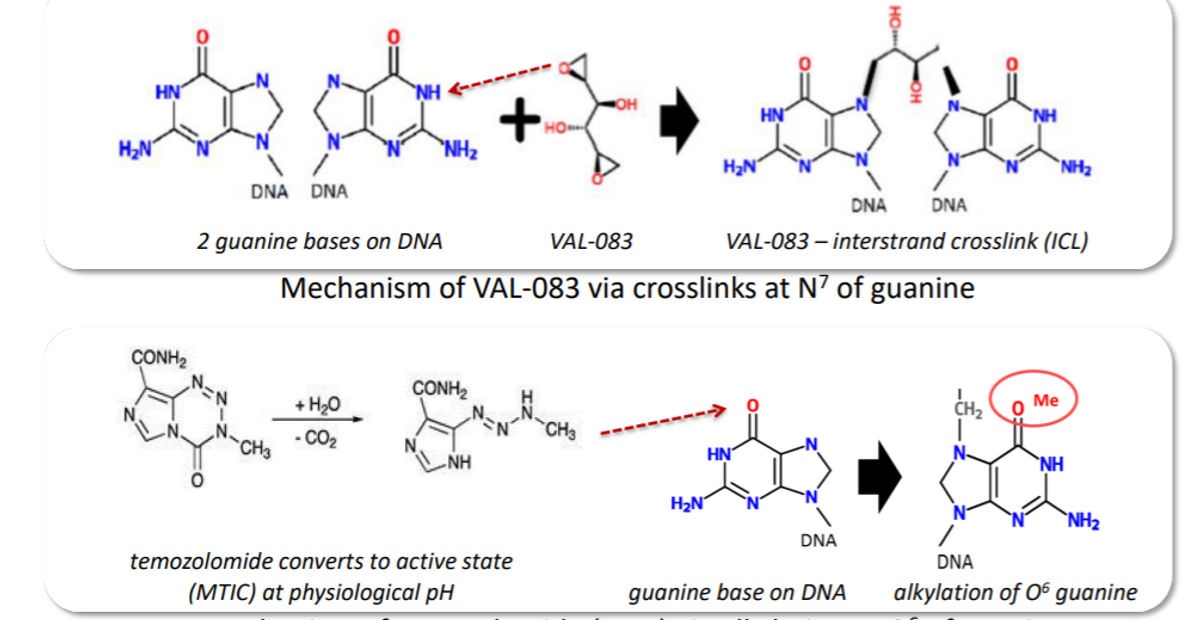
VAL-083 is a novel bi-functional DNA targeting agent that can induce interstrand cross-links at N7-guanine leading to DNA double-strand breaks, and finally, cell death. Unlike temozolomide, which is alkylating at 06 of guanine, VAL-083 is able to overcome the temozolomide resistance in vitro and in vivo. It acts as a radio-sensitizer against GBM cancer stem cells. Historical studies have shown that VAL-083, in combination with radiotherapy, improved patient survival. In this study, we designed it to use VAL-083 in combination with radiotherapy. It may offer a treatment alternative against glioblastoma with MGMT-mediated resistance to chemotherapeutic agents, including temozolomide.
The study was designed as follows: the patient received surgery and then radiation concurrent with VAL-083. The radiation dosage is 60 GY and the VAL-083 intravenous infusion is on days one, two, and three of a 21-day cycle. After radiotherapy, the patient received up to an additional eight cycles of VAL-083 treatment as maintenance therapy. As part of the dose-escalation phase, we used a starting dosage of 20mg then 30mg, and finally 40mg/m2/day to confirm MTDs. We then had the expansion phase of about 20 subjects at the MTD dosage which was the 30mg dose.
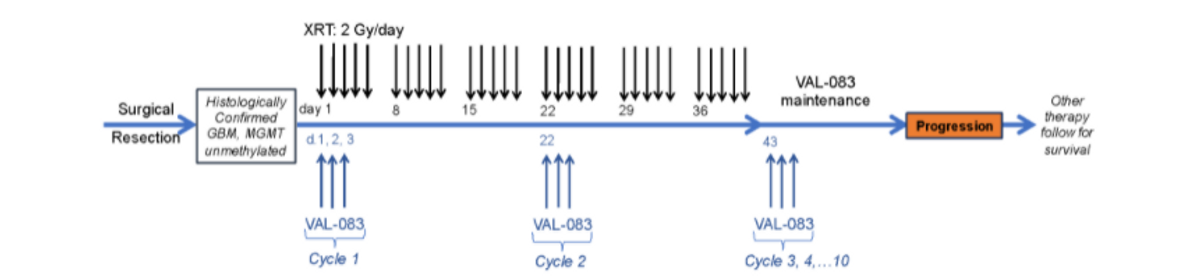
The endpoint for the escalation phase was safety and tolerability, and then for the expansion phase, it was PFS and OS.
This is the patient overview. The main inclusion criterion was that patients have newly diagnosed MGMT-unmethylated glioblastoma. After surgery, within seven weeks, patients received radiation and VAL-083 treatment. This is the patient total of 29 patients we enrolled.
For safety, we found dose-limiting toxicity in three cases. One was in the 40mg dosage, another two were at 30mg. In the 30mg dosage, one was hematological, another one was non-hematological. For all the patients, we found that most adverse events were myelosuppression and anemia, and all hematological AEs generally resolved spontaneously, so they were acceptable.
As for the pharmacokinetics in the blood, the graph is displayed here. The half-life of VAL-083 is about one hour. Initially, we get to the CSF two hours after infusion. We found the concentration in CSF is at least the same as in the plasma. That confirmed the ability of VAL-083 to reach the therapeutic target.
The best response assessed prior to cycle 4, for all the patients that started the study after their surgery and who had a measurable tumor at baseline, a total of 24 patients, was seven cases at about 30% complete response (CR). In the 30mg group, it was about 25% CR. Interestingly, in 30mg dosage group, because we have another five cases at the start that are below measurable level tumor size, so total is 10 patients in this dosage of the group, we get 10 patients that are CR, so CR percentage has reached 40%.
Continuing for the patients that received the cycles, we found the patients that received more than 10 cycles of VAL-083 get much better results as compared to less than 10 cycles. For the CR here and the stable disease (SD) cases in less than 10 cycles is 60 and 13%, but over 10 cycles SD is at 28, and CR which is at 71.
For the whole group of patients, the PFS was 9.3 months, which is much better than historical data. For the 30mg dose patients, the PFS was 8.7 months. For the OS, the same. The whole group reached almost 20 months. In 30mg, it’s similar, 19.1 months for the OS, so it's much better than historical results.
This is our case study; a female, 32 years old received surgery for the right temporal lobe tumor. Pathologically it's a GBM, with a wild type IDH, and MGMT-unmethylated.
The patient received radiation of 60 GY and the VAL-083 concurrent with radiation in two cycles with a 30mg dose. Then after they received intense treatment, a total of 11 cycles, 9 with 30mg, and another two with 20mg doses. The AE was very mild, with only Grade II myelosuppression. The patient, after follow-up last June, is still tumor-free, survived up to almost 40 months.
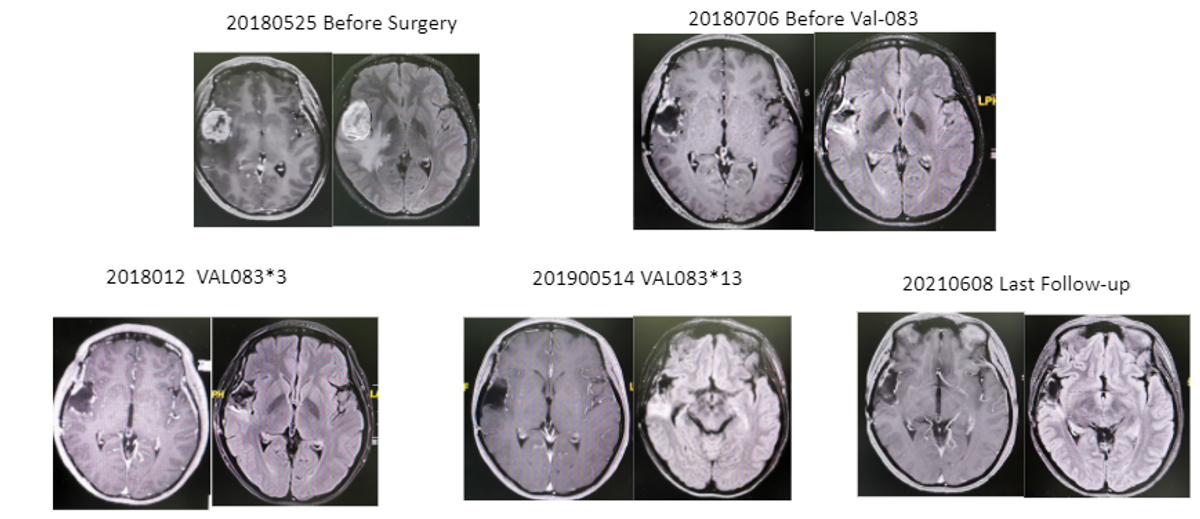
This is before surgery, after surgery, three cycles, 13 cycles after treatment, you see the last follow-up.
What we learned from this study. VAL-083 at 30mg/m2/day combined with radiotherapy is generally safe and well-tolerated, and we can use multiple treatment cycles, the median was nine. AE: the most common is myelosuppression. The VAL-083 levels in the CSF were found to be at least as high as in those in plasma. And VAL-083 at a 30mg dosage in combination with radiotherapy has demonstrated a benefit over historical standard-of-care in the same setting. VAL-083 now is being evaluated in the GBM AGILE study.
I want to thank our research team and also collaborators, and I'm happy to take any questions, thank you.
Located in San Diego, California, Kintara is dedicated to the development of novel cancer therapies for patients with unmet medical needs. Kintara is developing two late-stage, Phase 3-ready therapeutics for clear unmet medical needs with reduced risk development programs. The two programs are VAL-083 for GBM and REM-001 for CMBC. VAL-083 is a "first-in-class", small-molecule chemotherapeutic with a novel mechanism of action that has demonstrated clinical activity against a range of cancers, including central nervous system, ovarian and other solid tumors (e.g., NSCLC, bladder cancer, head and neck) in U.S. clinical trials sponsored by the National Cancer Institute (NCI). Based on Kintara's internal research programs and these prior NCI-sponsored clinical studies, Kintara is currently conducting clinical trials to support the development and commercialization of VAL-083 in GBM. Kintara is also advancing its proprietary, late-stage photodynamic therapy platform that holds promise as a localized cutaneous, or visceral, tumor treatment as well as in other potential indications. REM-001 therapy, has been previously studied in four Phase 2/3 clinical trials in patients with CMBC, who had previously received chemotherapy and/or failed radiation therapy. With clinical efficacy to date of 80% complete responses of CMBC evaluable lesions, and with an existing robust safety database of approximately 1,100 patients across multiple indications, Kintara is advancing the REM-001 CMBC program to late-stage pivotal testing. SAFE
HARBOR STATEMENT
Any statements contained in this press release that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995, including statements regarding the status of the Company's clinical trials and the GBM AGILE study. Any forward-looking statements contained herein are based on current expectations but are subject to a number of risks and uncertainties. The factors that could cause actual future results to differ materially from current expectations include, but are not limited to, risks and uncertainties relating to the impact of the COVID-19 pandemic on the Company's operations and clinical trials; the Company's ability to develop, market and sell products based on its technology; the expected benefits and efficacy of the Company's products and technology; the availability of substantial additional funding for the Company to continue its operations and to conduct research and development, clinical studies and future product commercialization; and, the Company's business, research, product development, regulatory approval, marketing and distribution plans and strategies. These and other factors are identified and described in more detail in the Company's filings with the SEC, including the Company's Annual Report on Form 10-K for the year ended June 30, 2021, the Company's Quarterly Reports on Form 10-Q, and the Company's Current Reports on Form 8-K.
For more information, please visit www.kintara.com or follow us on Twitter at @Kintara_Thera, Facebook and Linkedin.