SAN DIEGO, Sept. 22, 2021 -- Kintara Therapeutics, Inc. (Nasdaq: KTRA) ("Kintara" or the "Company"), a biopharmaceutical company developing novel cancer therapies for patients who are failing, or are resistant to, current treatment regimens today announced topline data results from the newly-diagnosed adjuvant arm of its open-label, Phase 2 clinical study being conducted at the MD Anderson Cancer Center (MD Anderson) in Houston, Texas.
The Phase 2 trial was a two-arm, biomarker-driven study testing VAL-083 in glioblastoma multiforme (GBM) patients who have an unmethylated promoter of the methylguanine DNA-methyltransferase (MGMT) gene. The Company previously announced (July 2021) topline data results from the recurrent GBM arm of the study which provided important safety and efficacy data to support the continued evaluation of VAL-083 as a treatment option for GBM.
The newly-diagnosed adjuvant arm of the study addressed GBM patients requiring adjuvant therapy after chemoradiation with temozolomide. The trial arm enrolled 39 patients (36 efficacy evaluable) initially receiving a dose of 30 mg/m2/day on days 1, 2 and 3 of a 21-day cycle.
Summary of results:
- Progression Free Survival (PFS) for the 36 efficacy evaluable patients is 10.0 months (95% Confidence Interval (CI) 8.2-10.8 months). While this is not a head-to-head trial, historical data for this patient population has demonstrated PFS of 5.3-6.9 months*.
- Median overall survival (mOS) for the 36 efficacy evaluable patients is 16.5 months (CI 13.3-19.3 months). While this is not a head-to-head trial historical data for this patient subpopulation has demonstrated mOS of 12.7-16.0 months*.
- Consistent with prior studies, myelosuppression was the most common adverse event. One patient experienced a serious adverse event (SAE) possibly related to VAL-083.
The dosing regimen (30 mg/m2/day) of the MD Anderson study mirrors the trial design of the newly-diagnosed adjuvant study arm of the GBM AGILE study. GBM AGILE, which is sponsored by the Global Coalition for Adaptive Research (GCAR), is a revolutionary, patient-centered, registrational, seamless Phase 2/3 adaptive platform trial evaluating multiple therapies for patients with newly-diagnosed and recurrent GBM. VAL-083 currently represents the only therapeutic agent being evaluated in all three GBM patient subtypes: methylated MGMT, newly-diagnosed unmethylated MGMT, and recurrent.
"On behalf of the entire Kintara team, I wish to extend gratitude to MD Anderson, and all of the patients who participated in both arms of the trial," said Saiid Zarrabian, Kintara's Chief Executive Officer. "The topline results from the newly-diagnosed adjuvant arm are a particularly important milestone for the company as it further affirms the efficacy and safety data reported this past July from the recurrent arm, thus providing additional support and momentum to continue the evaluation of VAL-083 for the treatment of GBM."
Dr. Barbara O'Brien, the Principal Investigator for the Phase 2 study at MD Anderson added, "I continue to be impressed by the clinical data generated by both arms of the study and remain excited by VAL-083's potential to be a game-changing therapeutic agent to help patients suffering from this deadly disease."
VAL-083 is independent of the MGMT resistance mechanism and has been assessed in over 40 Phase 1 and Phase 2 clinical trials in multiple indications sponsored by the U.S. National Cancer Institute (NCI). Published pre-clinical and clinical data indicate that VAL-083 has activity against a range of tumor types, including lung, brain, cervical, ovarian tumors and hematologic (blood) cancers. VAL-083 has been granted Orphan Drug Designation for GBM by the FDA and EMA and has also been granted Orphan Drug Designations for medulloblastoma and ovarian cancer by the FDA. In addition, the FDA has granted Fast Track Designation for VAL-083 in recurrent GBM. VAL-083 is approved as a cancer chemotherapeutic in China for the treatment of chronic myelogenous leukemia and lung cancer. VAL-083 has not been approved for any indications outside of China.
* Hegi et al N Eng J Med 352; 997-1003 (2005);
Tanguturi et al. NeuroOncol. 19(7): 908-917 (2017)

SAN DIEGO, Sept. 28, 2021 /PRNewswire/ -- Kintara Therapeutics, Inc. (Nasdaq: KTRA) ("Kintara" or the "Company"), a biopharmaceutical company developing novel cancer therapies for patients who are failing, or are resistant to, current treatment regimens today announced the closing of its previously announced registered direct offering, priced at-the-market under Nasdaq rules, with several healthcare-focused institutional investors of 12,000,000 shares of its common stock (or common stock equivalents) and warrants to purchase up to an aggregate of 12,000,000 shares of common stock, at a combined offering price of $1.25 per share and associated warrant. The warrants have an exercise price of $1.25 per share and are exercisable for three and one half years from the date of issuance. The gross proceeds to the Company totaled approximately $15 million, before deducting placement agent fees and offering expenses.
H.C. Wainwright & Co. acted as the exclusive placement agent for the offering.
The Company currently intends to use the net proceeds from the offering for funding its clinical studies, working capital and other general corporate purposes, including, but not limited to, funding acquisitions or investments in businesses, products or technologies that are complementary to the Company's businesses, products and technologies.

SAN DIEGO, Sept. 29, 2021 /PRNewswire/ -- https://www.kintara.com/ (Nasdaq: KTRA) ("Kintara" or the "Company"), a biopharmaceutical company focused on the development of new solid tumor cancer therapies, today announced financial results for its fiscal year ended June 30, 2021 and provided a corporate update.
CORPORATE HIGHLIGHTS AND RECENT DEVELOPMENTS
- Entered into securities purchase agreements with healthcare-focused institutional investors to raise approximately $15 million in gross proceeds (September). Funding from this registered direct offering, which was priced at a premium to market, was consummated on September 28, 2021, and provides cash for ongoing clinical studies and corporate working capital needs.
- Bolstered patient enrollment opportunities in the U.S. by activating additional clinical trial sites for glioblastoma (GBM) patients for the VAL-083 arm of the GBM AGILE registrational study sponsored by the Global Coalition for Adaptive Research (GCAR).
- Announced initiation of patient recruitment at first site (January)
- Reported activation of 15 clinical sites for the GBM AGILE study (May)
- Updated site activation to 26 clinical sites (August)
The GBM AGILE study is a revolutionary, patient-centered, adaptive platform trial for registration evaluating multiple therapies for patients with newly-diagnosed and recurrent GBM. VAL-083 currently represents the only therapeutic agent being evaluated in all subgroups of GBM AGILE (newly-diagnosed methylated MGMT, newly-diagnosed unmethylated MGMT, and recurrent patients). The study will enroll up to 150 patients in the initial evaluation (labeled 'stage 1') for the VAL-083 arm of the study at over 40 sites in the U.S. and Canada, with potential to increase to 65 clinical trial centers worldwide. The Company is forecasting that the recent financing will provide sufficient funding through stage 1, which could result in graduation to the final confirmatory stage, the potentially NDA enabling portion of the GBM AGILE study.
- Reported topline results from the Phase 2 study conducted at the MD Anderson Cancer Center that affirm the safety and efficacy of VAL-083 in two different GBM patient subtypes and support continued evaluation of VAL-083 in the GBM AGILE registrational study.
- In September, topline Phase 2 clinical study results for VAL-083 as adjuvant therapy for newly-diagnosed GBM patients were reported demonstrating progression free survival (PFS) and overall survival (OS) of 10.0 months and 16.5 months, respectively, in efficacy evaluable patients
- In July, topline Phase 2 clinical study results for VAL-083 for recurrent GBM were reported demonstrating median overall survival (mOS) for the 48 efficacy evaluable patients initially receiving the GBM AGILE treatment dose of 30 mg/m2/day of 8.0 months.
- Continued to advance development of REM-001 for the treatment of Cutaneous Metastatic Breast Cancer (CMBC), including taking critical steps toward manufacturing sufficient quantity of drug to allow for initiation and completion of our 15-patient lead-in CMBC study.
- Joined the Russel Microcap Index effective June 28, 2021. Russell Indexes are widely used by investment managers and institutional investors for index funds and as benchmarks for active investment strategies. Approximately $10.6 trillion in assets are benchmarked against Russell's U.S. Indexes.
- Enhanced leadership team by appointing Tamara A. Seymour to the Board of Directors (April). Ms. Seymour is a corporate finance veteran with three decades of experience in biotech and life sciences including roles as a Chief Financial Officer and Board member of publicly-listed companies.
"As we embark on a new fiscal year with a strengthened cash position from our recent financing, I'm extremely pleased with where the Company is positioned on the clinical and corporate development fronts," commented Saiid Zarrabian, Kintara's President and Chief Executive Officer. "Moving forward, our diversified, late-stage pipeline has multiple, significant near-term milestones, highlighted by the GCAR GBM AGILE study. We believe this registration study represents an extraordinary opportunity for the Company as it provides an optimal clinical path given its highly accelerated program as evidenced by the initiation of patient enrollment at 26 sites in less than eight months, and from a cost savings standpoint through an FDA approved registrational trial which provides Kintara the unique opportunity to enroll three separate GBM patient subtypes. We are entering a pivotal juncture in the Company's development, and I wish to extend gratitude to our longstanding shareholders for their continued support."

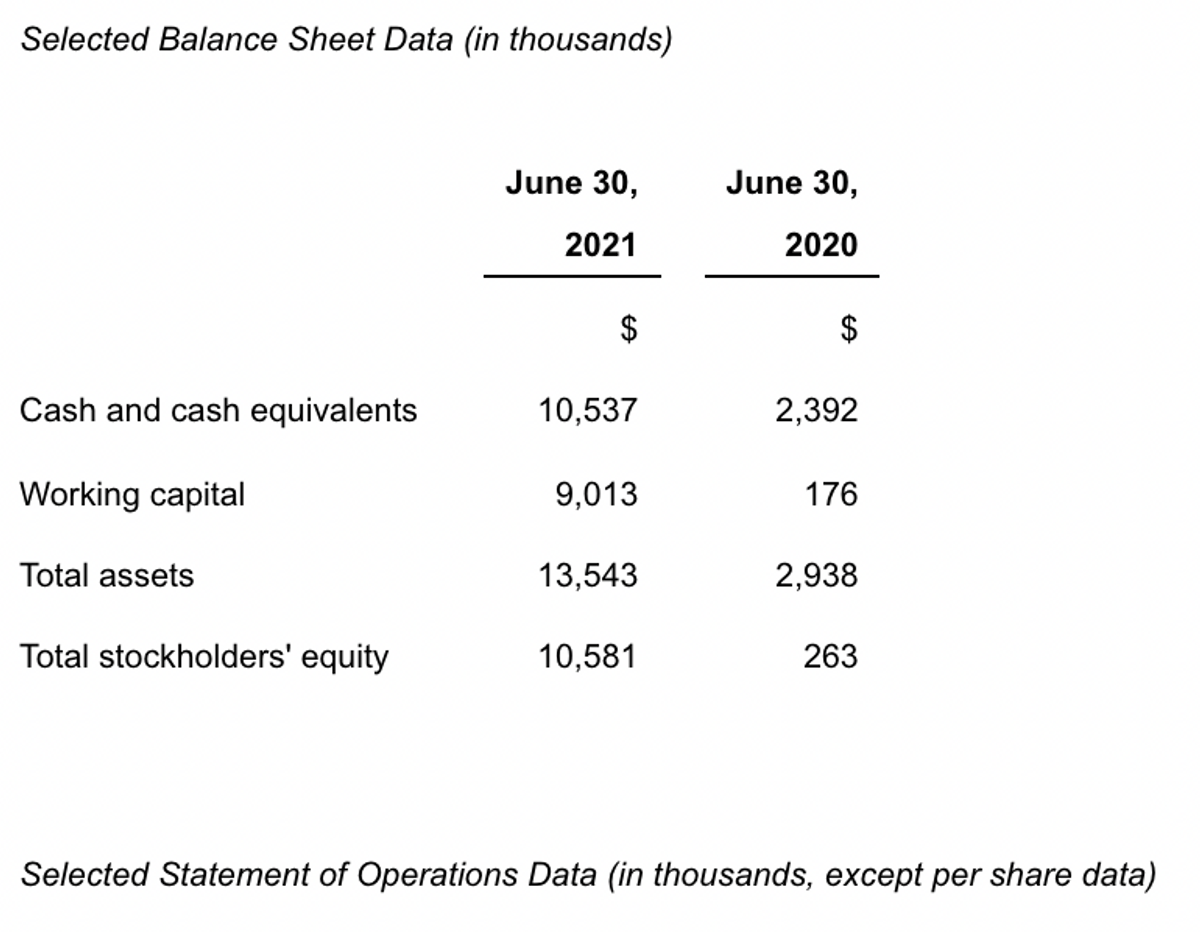
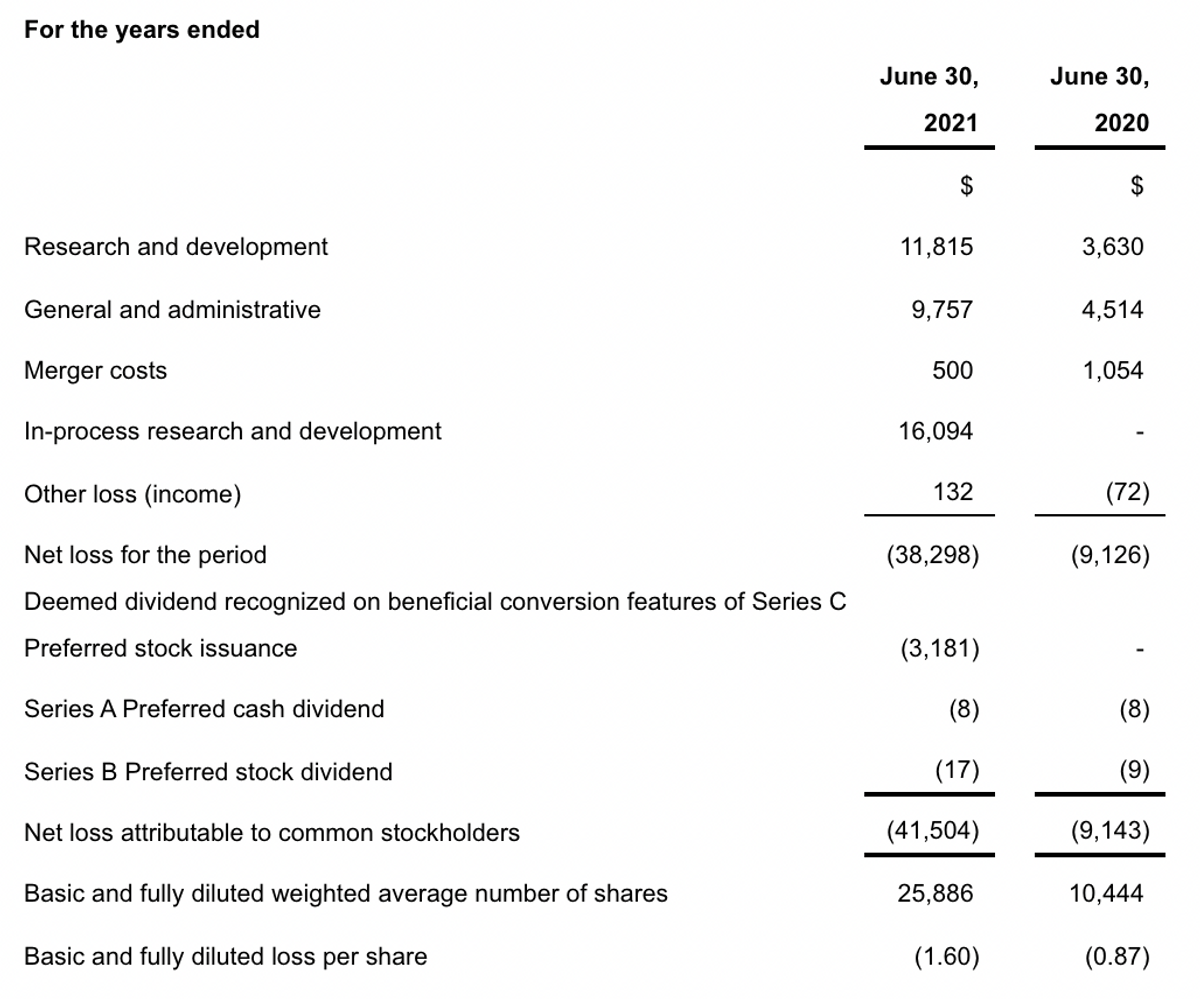
On June 30, 2021, the Company had cash and cash equivalents of approximately $10.5 million. For the year ended June 30, 2021, the Company reported a net loss of approximately $38.3 million, or $1.60 per share, compared to a net loss of approximately $9.1 million, or $0.87 per share, for the year ended June 30, 2020. The increase in loss for the year ended June 30, 2021 compared to the year ended June 30, 2020, was largely due to the recognition of $16.1 million of non-cash expenses related to the acquisition of in-process research and development costs associated with the acquisition of Adgero Biopharmaceuticals Holdings, Inc. and an expanded rate of expenditures with the initiation of the GCAR study and REM-001 development.
Kintara's financial statements as filed with the U.S. Securities Exchange Commission can be viewed on the Company's website at: http://ir.kintara.com/sec-filings.
Located in San Diego, California, Kintara is dedicated to the development of novel cancer therapies for patients with unmet medical needs. Kintara is developing two late-stage, Phase 3-ready therapeutics for clear unmet medical needs with reduced risk development programs. The two programs are VAL-083 for GBM and REM-001 for CMBC. VAL-083 is a "first-in-class", small-molecule chemotherapeutic with a novel mechanism of action that has demonstrated clinical activity against a range of cancers, including central nervous system, ovarian and other solid tumors (e.g., NSCLC, bladder cancer, head and neck) in U.S. clinical trials sponsored by the National Cancer Institute (NCI). Based on Kintara's internal research programs and these prior NCI-sponsored clinical studies, Kintara is currently conducting clinical trials to support the development and commercialization of VAL-083 in GBM. Kintara is also advancing its proprietary, late-stage photodynamic therapy platform that holds promise as a localized cutaneous, or visceral, tumor treatment as well as in other potential indications. REM-001 therapy, has been previously studied in four Phase 2/3 clinical trials in patients with CMBC, who had previously received chemotherapy and/or failed radiation therapy. With clinical efficacy to date of 80% complete responses of CMBC evaluable lesions, and with an existing robust safety database of approximately 1,100 patients across multiple indications, Kintara is advancing the REM-001 CMBC program to late-stage pivotal testing. SAFE
HARBOR STATEMENT
Any statements contained in this press release that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995, including statements regarding the status of the Company's clinical trials and the GBM AGILE study. Any forward-looking statements contained herein are based on current expectations but are subject to a number of risks and uncertainties. The factors that could cause actual future results to differ materially from current expectations include, but are not limited to, risks and uncertainties relating to the impact of the COVID-19 pandemic on the Company's operations and clinical trials; the Company's ability to develop, market and sell products based on its technology; the expected benefits and efficacy of the Company's products and technology; the availability of substantial additional funding for the Company to continue its operations and to conduct research and development, clinical studies and future product commercialization; and, the Company's business, research, product development, regulatory approval, marketing and distribution plans and strategies. These and other factors are identified and described in more detail in the Company's filings with the SEC, including the Company's Annual Report on Form 10-K for the year ended June 30, 2021, the Company's Quarterly Reports on Form 10-Q, and the Company's Current Reports on Form 8-K.
For more information, please visit www.kintara.com or follow us on Twitter at @Kintara_Thera, Facebook and Linkedin.