Glioblastoma (GBM) is the most common and deadliest form of brain cancer. Standard treatments for GBM include surgery, radiation, and the chemotherapy agent temozolomide (TMZ). TMZ works by alkylating DNA which causes damage to create single-strand breaks in the DNA that results, if left unrepaired in the death of the cancer cell. TMZ largely alkylates the O6 position of guanine residues found in all DNA, a broad mechanism that hits healthy cells as well, but is particularly effective in targeting rapidly dividing cells, like the malignant cells in brain tumors.
Even after the standard therapies, nearly all GBM tumors recur and as a result, the 5-year patient survival rate is less than 5%, largely due to the development of chemoresistance to TMZ. Chemoresistance occurs because tumor cells are able to repair the DNA damage caused by TMZ through an enzyme called O6-methylguanine DNA methyltransferase (MGMT), which is encoded by the MGMT gene. Accordingly, the presence of high MGMT enzyme activity in brain tumors predicts poor response to TMZ. Regulation of the MGMT gene is controlled by epigenetic silencing through methylation, whereby the unmethylated promoter results in high levels of the TMZ-neutralizing MGMT enzyme. Many patients develop this resistance over time, but potentially as many as 60% of GBM patients are diagnosed with this unmethylated promoter, and these are the patients that are inherently resistant to TMZ with the highest unmet need for improved therapies.

Recent Kintara presentations at the American Association for Cancer Research (AACR) and the Society for Neuro-Oncology (SNO) annual meetings have provided further evidence on the unique and potentially breakthrough mechanism of action of Kintara’s VAL-083 for treating GBM patients.
VAL-083 is a novel bi-functional DNA targeting agent with a mechanism of action distinct from TMZ and other older generation DNA damaging agents such as lomustine and carmustine. There are key differences that make the drug particularly attractive for GBM patients. First, VAL-083’s activity largely results in interstrand cross-links at the N7-guanine of DNA, which is different from the O6-guanine target for TMZ. Importantly, these cross-links result in double-strand breaks (DSBs) which are not repairable by the MGMT enzyme. As such, VAL-083’s unique cytotoxic mechanism circumvents MGMT-mediated chemoresistance and also maintains cytotoxic activity in cancer cells deficient in the DNA mismatch repair (MMR) process (another drug resistance mechanism) (see figure 1 & 2 below).
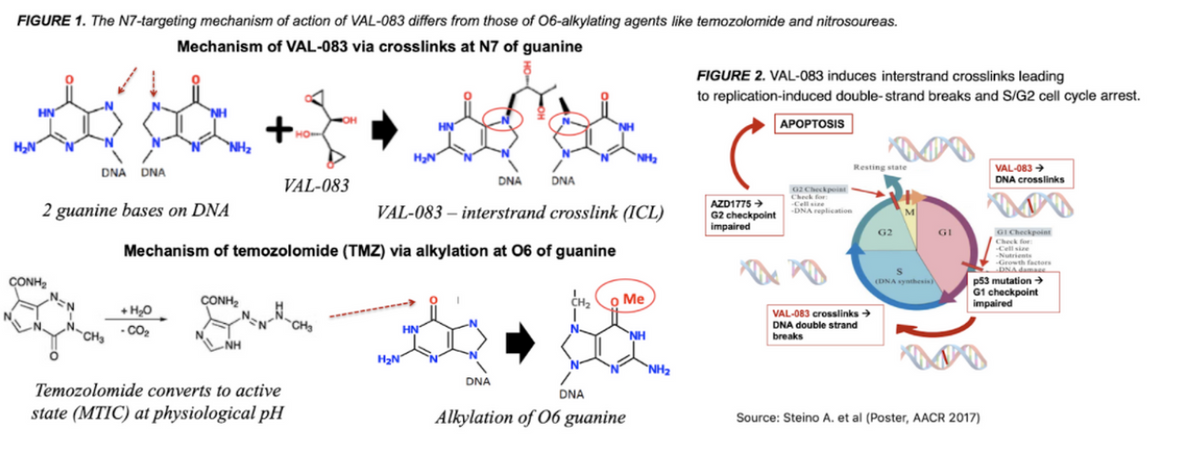
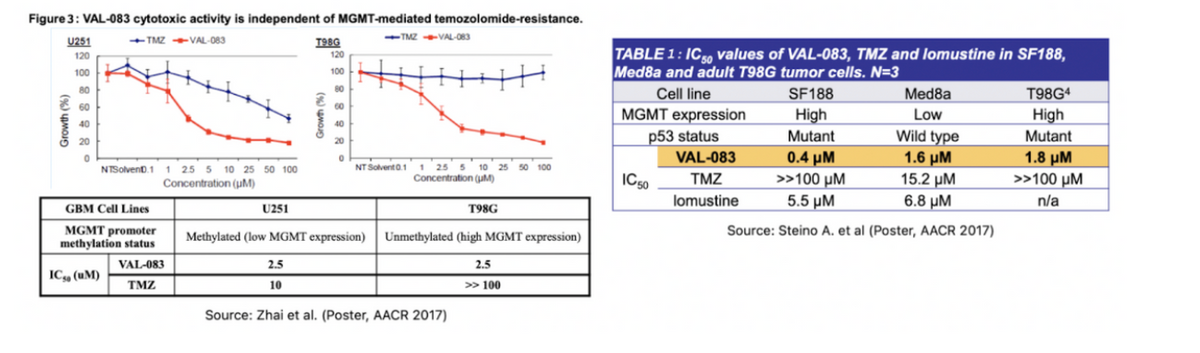
Secondly, VAL-083 is a highly potent cytostatic and cytotoxic agent with a rapid onset of action. IC50 data in the figure and table below show greater activity in various cell lines compared to both TMZ and lomustine, with cytotoxic activity independent of MGMT-promoter status. So, VAL-083 is designed to work as well as TMZ in methylated patients and offers an effective option in unmethylated patients where TMZ has a limited effect.

VAL-083 has the potential to give physicians and GBM patients a critical alternative chemotherapy option for treating GBM patients independent of MGMT promoter status. Currently, chemotherapy remains the only option for GBM patients because, to date, treating GBM with targeted therapy such as small molecule tyrosine kinase inhibitors (TKIs) or targeted monoclonal antibodies such as immune checkpoint inhibitors has been overwhelmingly disappointing.
See Westphal M. et al., EGFR as a Target for Glioblastoma Treatment: An Unfulfilled Promise, CNS Drugs, 2017.
Failures of targeted therapy have largely been due to poor brain penetration of these drugs across the blood brain barrier (BBB). The BBB is a highly selective semipermeable physical border of endothelial cells that prevents most substances in the circulating blood from non-selectively crossing into the cerebrospinal fluid (CSF) of the central nervous system. While the BBB is critical for preventing harmful agents like pathogens, toxins, and circulating immune cells from getting into the brain, it works against patients with GBM because it also limits the penetration of small molecule therapeutics and biologics such as monoclonal antibodies that are commonly used and are effective in treating cancer in other parts of the body.
One monoclonal antibody that is approved for GBM is Roche’s Avastin® (bevacizumab). The mechanism of action for Avastin is in blocking vascular endothelial growth factor (VEGF). VEGF is a signaling protein that promotes the growth of new blood vessels. The rationale was that Avastin could help prevent cancerous cells in the brain from proliferating and spreading in the brain by limiting oxygen supply due to poor tumor vasculature. Avastin does show modest improvement in slowing GBM disease progression, but clinical trials have not resulted in an increase in overall survival. Again, this is likely due in part to the drug’s poor BBB penetration.
This is also potentially why immunotherapies, such as Merck’s Keytruda® (pembrolizumab) and Bristol Myers Squibbs’ Opdivo® (nivolumab), two monoclonal antibodies, have demonstrated very limited activity in GBM. They also do not cross the BBB in sufficient concentrations. Additionally, the brain, which was previously thought to be immune-privileged, does have some immune cells, but the lack of highly infiltrating lymphocytes probably suggests that brain tumor cells do not overly express immune checkpoints, such as PD-L1. Additionally, the lack of antigen-presenting cells in the brain also may contribute to why many GBM immunotherapies have failed.
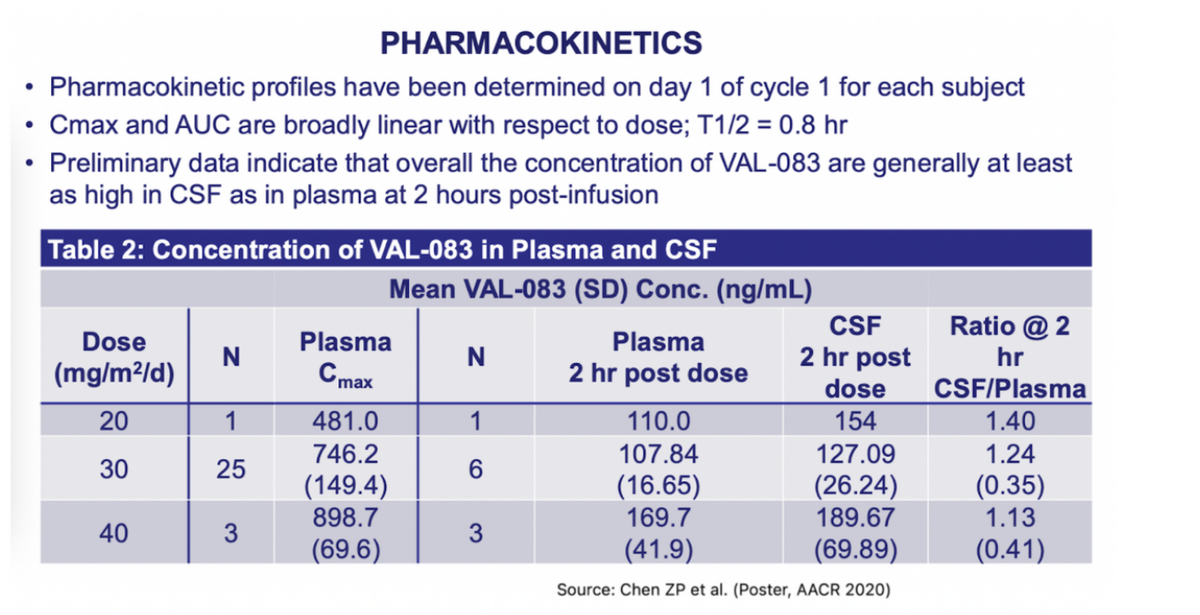
TMZ has been shown to cross the BBB with about 20% of the levels when compared to blood plasma levels. That level is enough to show responses in some GBM tumors. VAL-083 has excellent penetration across the BBB. Pharmacokinetic data presented at the AACR meeting in 2020 show that VAL-083 readily crosses the blood brain barrier and accumulates in brain tumor tissue at concentrations at least as high as in plasma (see table 2 below). The efficient BBB penetration of VAL- 083 along with its highly potent cytotoxic activity has generally been well-tolerated by patients.
(Table 2)

Throughout 2021, Kintara has been investigating VAL-083 in two open-label Phase 2 clinical studies. One of the studies was at the Sun Yat-sen University Cancer Center in China, and the second study was at MD Anderson Cancer Cancer in the U.S. The most recent data updates from these studies were presented at the SNO meeting in November 2021.
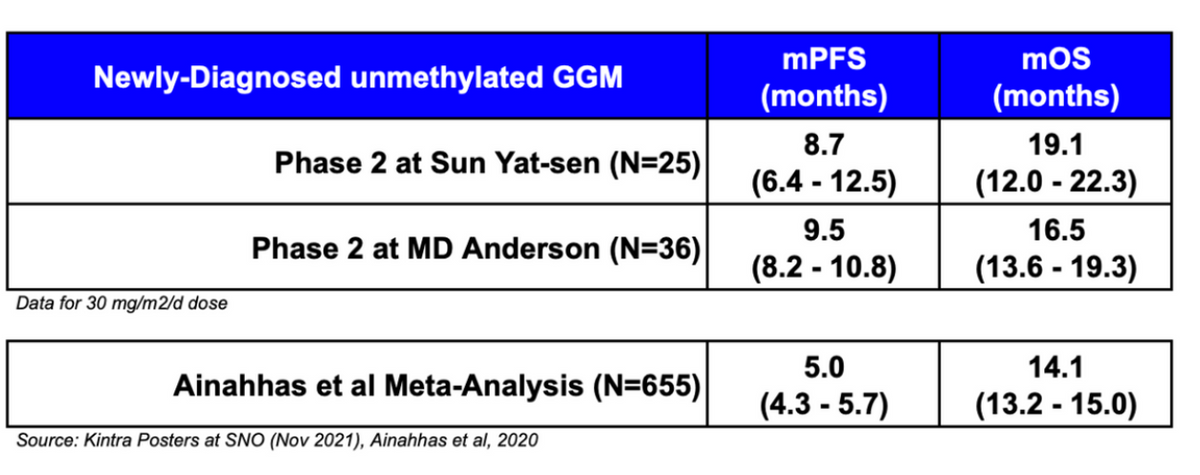
The study at Sun Yat-sen enrolled newly-diagnosed, first-line patients. A total of 29 patients have been treated in the study, 25 of whom received a starting dose of 30 mg/m2/day - a dose which investigators have reported to be generally safe and well-tolerated in combination with radiation therapy, with many patients completing multiple treatment cycles in the first-line setting. Results for the 30 mg/m2/day patients show a progression-free survival (PFS) of 8.7 months (95% CI: 6.4-12.5) and a median overall survival (mOS) of 19.1 months (95% CI: 12.0-22.3) (see SNO 2021 Poster CTNI21).
The study at MD Anderson enrolled patients in both the adjuvant and recurrent settings. Because VAL-083 works distinctly different from TMZ, adjuvant and recurrent patients might benefit from the drug, especially the unmethylated MGMT patients. A total of 39 patients (36 evaluable) were enrolled in the adjuvant setting and 89 (83 evaluable) were enrolled with recurrent disease. In the adjuvant setting, data show a PFS of 9.5 months (95% CI: 8.2-10.8) and a mOS of 16.5 months (95% CI: 13.6-19.3). In the recurrent setting, data showed a mOS of 8.0 months (95% CI: 6.6-10.3) for the optimal 30 mg/m2/day dose (see SNO 2021 Poster CTNI26).
These data compare well to historical control studies examining GBM patients in the first-line setting. For example, a systematic review and meta-analysis on the benefits of TMZ were published by a group from the Division of Neuro-Oncology, Department of Neurology, Thomas Jefferson University in Philadelphia, PA (Alnahhas I et al., 2020). The group looked at the effects of TMZ on newly-diagnosed GBM patients with both MGMT methylated and unmethylated promoter.
In the methylated GBM cohort, data pooled from 7 studies (N=805) show a median PFS of 9.5 months (95% CI: 7.4-11.6) and mOS of 24.6 months (95% CI: 22.2-27.0). In contrast, data from 5 studies (N=655) in GBM patients with unmethylated MGMT promoter showed a median PFS of only 5.0 months (95% CI: 4.3-5.7) and mOS of 14.1 months (95% CI: 13.2-15.0).
Below is a summary of the data from the two Phase 2 studies and the Alnahhas I et al., meta-analysis. The observed benefit for VAL-083 to date is very encouraging, showing mOS improvement over historical clinical data.

The attractiveness of VAL-083’s mechanism of action and the data generated to date was impressive enough for The Global Coalition for Adaptive Research (GCAR) to announce in October 2020 that VAL-083 would be included in its landmark Phase 2/3 clinical study called GBM AGILE, an adaptive study designed to evaluate multiple therapies in newly-diagnosed and recurrent GBM patients. The obvious patient subtype of particular interest is the unmethylated MGMT GBM population where there is no effective standard-of-care and VAL-083 shows significant promise.
Furthermore, because GBM AGILE is a coalition study, the overhead costs are shared. Two other companies, Bayer and Kazia Therapeutics, have also been selected to participate in the study. The principal investigators for Kintara are Dr. John de Groot, Division Chief Neuro-Oncology Division, Department of Neurological Surgery, University of California, San Francisco, and Dr. James Perry, Professor of Neurology at the University of Toronto and Sunnybrook Research Institute. Enrollment is taking place at top-academic and medical centers in the U.S. and Canada. The primary endpoint is overall survival (OS). This is a fantastic opportunity for Kintara because it reduces costs, saves time, and provides a high level of confidence in the management of the trials.

In summary, Glioblastoma Multiforme is a significant unmet medical need. The five-year survival rate is less than 5%. The current approved first-line treatment, temozolomide (TMZ), was approved in 1999 and has been generic since 2010.
GBM is currently an $800 million market and is expected to grow to $1.4 billion by 2027 (GlobalData, November 2018).
While VAL-083 works in a similar manner to TMZ in that both are DNA damaging agents that result in DNA strand breaks in cancer cells, resistance to TMZ is high due to MGMT DNA repair as well as MMR mechanisms that counter the activity of TMZ. The highest unmet medical need is in the approximate 60% of GBM patients who possess up-regulated activity of the MGMT repair enzyme, and this is where VAL-083 has shown impressive potential in the recently completed Phase 2 clinical studies.
Located in San Diego, California, Kintara is dedicated to the development of novel cancer therapies for patients with unmet medical needs. Kintara is developing two late-stage, Phase 3-ready therapeutics for clear unmet medical needs with reduced risk development programs. The two programs are VAL-083 for GBM and REM-001 for Cutaneous Metastatic Breast Cancer (CMBC). VAL-083 is a "first-in-class", small-molecule chemotherapeutic with a novel mechanism of action that has demonstrated clinical activity against a range of cancers, including central nervous system, ovarian and other solid tumors (e.g., NSCLC, bladder cancer, head and neck) in U.S. clinical trials sponsored by the National Cancer Institute (NCI). Based on Kintara's internal research programs and these prior NCI-sponsored clinical studies, Kintara is currently conducting its GBM AGILE study to support the development and commercialization of VAL-083 in GBM.
Kintara is also advancing its proprietary, late-stage photodynamic therapy platform that holds promise as a localized cutaneous, or visceral, tumor treatment as well as in other potential indications. REM-001 therapy, has been previously studied in four Phase 2/3 clinical trials in patients with CMBC, who had previously received chemotherapy and/or failed radiation therapy. With clinical efficacy to date of 80% complete responses of CMBC evaluable lesions, and with an existing robust safety database of approximately 1,100 patients across multiple indications, Kintara is advancing the REM-001 CMBC program to late-stage pivotal testing.
HARBOR STATEMENT
Any statements contained in this press release that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995, including statements regarding the status of the Company's clinical trials and the GBM AGILE study. Any forward-looking statements contained herein are based on current expectations but are subject to a number of risks and uncertainties. The factors that could cause actual future results to differ materially from current expectations include, but are not limited to, risks and uncertainties relating to the impact of the COVID-19 pandemic on the Company's operations and clinical trials; the Company's ability to develop, market and sell products based on its technology; the expected benefits and efficacy of the Company's products and technology; the availability of substantial additional funding for the Company to continue its operations and to conduct research and development, clinical studies and future product commercialization; and, the Company's business, research, product development, regulatory approval, marketing and distribution plans and strategies. These and other factors are identified and described in more detail in the Company's filings with the SEC, including the Company's Annual Report on Form 10-K for the year ended June 30, 2021, the Company's Quarterly Reports on Form 10-Q, and the Company's Current Reports on Form 8-K.
For more information, please visit www.kintara.com or follow us on Twitter at @Kintara_Thera, Facebook and Linkedin.










